American Journal of Medical Genetics Doi 10.1002 Ajmg.a - perrodinshandid
Angiotensinogen (AGT) gene missense polymorphisms (rs699 and rs4762) and diabetic nephropathy in Caucasians with type 2 diabetes mellitus
- Jana Makuc Department of Internal Medicine, General Hospital Slovenj Gradec, Slovenj Gradec, Slovenia
- Maja Šeruga Department of Internal Medicine, General Hospital Murska Sobota, Murska Sobota, Slovenia
- Matej Završnik Department of Endocrinology and Diabetology, Division of Internal Medicine, University Medical Centre Maribor, Maribor, Slovenia
- Ines Cilenšek Institute of Histology and Embryology, Faculty of Medicine, University of Ljubljana, Ljubljana, Slovenia
- Daniel Petrovič Institute of Histology and Embryology, Faculty of Medicine, University of Ljubljana, Ljubljana, Slovenia
Keywords: Angiotensinogen, AGT, rs699, rs4762, diabetic nephropathy, T2DM, type 2 diabetes mellitus
Abstract
Gene polymorphisms associated with the renin–angiotensin–aldosterone system (RAAS) have been extensively studied in diabetic nephropathy (DN) patients, due to therapeutic potential of targeting the RAAS and slowing down the disease progression. The aim of our study was to examine the association between angiotensinogen (AGT) gene polymorphisms (rs699 and rs4762) and DN in Caucasians with type 2 diabetes mellitus (T2DM). A total of 651 unrelated Slovenian (Caucasian) T2DM patients were tested for AGT rs699 and rs4762 polymorphisms using a novel fluorescence-based kompetitive allele-specific polymerase chain reaction (KASPar) assay. A study group consisted of 276 T2DM patients with DN, while control group included 375 patients without DN but who have had T2DM for >10 years. For rs699 polymorphism, the frequencies of GG, GA and AA genotypes were 20.6%, 52.2% and 27.2%, respectively in T2DM patients and 23.4%, 48.1% and 28.5%, respectively in controls. The distributions of GG, GA and AA genotypes for rs4762 polymorphism were 73.9%, 23.2% and 2.9%, respectively in T2DM patients and 70.4%, 27.5% and 2.1%, respectively in controls. No significant differences in the allele frequencies were found between T2DM patients and controls for both polymorphisms. AGT rs699 and rs4762 missense polymorphisms are not associated with DN in our subset of Slovenian T2DM patients.
Downloads
Download data is not yet available.
Author Biographies
Jana Makuc, Department of Internal Medicine, General Hospital Slovenj Gradec, Slovenj Gradec, Slovenia
Department of Internal Medicine
Maja Šeruga, Department of Internal Medicine, General Hospital Murska Sobota, Murska Sobota, Slovenia
Department of Internal Medicine
Matej Završnik, Department of Endocrinology and Diabetology, Division of Internal Medicine, University Medical Centre Maribor, Maribor, Slovenia
Department of Endocrinology and Diabetology
References
Gross JL, de Azevedo MJ, Silveiro SP, Canani LH, Caramori ML, Zelmanovitz T. Diabetic nephropathy: Diagnosis, prevention, and treatment. Diabetes Care 2005;28(1):164-76. https://doi.org/10.2337/diacare.28.1.164.
Yacoub R, Campbell KN. Inhibition of RAS in diabetic nephropathy. Int J Nephrol Renovasc Dis 2015;8:29-40. DOI: 10.2147/IJNRD.S37893.
Gnudi L, Goldsmith D. Renin angiotensin aldosterone system (RAAS) inhibitors in the prevention of early renal disease in diabetes. F1000 Rep 2010;2:18.
DOI: 10.3410/M2-18.
Shlipak M. Diabetic nephropathy. BMJ Clin Evid 2009;2009. pii:0606.
Carpena MP, Rados DV, Sortica DA, Souza BM, Reis AF, Canani LH, et al. Genetics of diabetic nephropathy. Arq Bras Endocrinol Metabol 2010;54(3):253-61. https://doi.org/10.1590/S0004-27302010000300002.
Kato M, Natarajan R. Diabetic nephropathy - Emerging epigenetic mechanisms. Nat Rev Nephrol 2014;10(9):517-30. https://doi.org/10.1038/nrneph.2014.116.
Van Buren PN, Toto R. Hypertension in diabetic nephropathy: Epidemiology, mechanisms, and management. Adv Chronic Kidney Dis 2011;18(1):28-41. https://doi.org/10.1053/j.ackd.2010.10.003.
Ahmad J. Management of diabetic nephropathy: Recent progress and future perspective. Diabetes Metab Syndr 2015;9(4):343-58. https://doi.org/10.1016/j.dsx.2015.02.008.
Rahimi Z, Moradi M, Nasri H. A systematic review of the role of renin angiotensin aldosterone system genes in diabetes mellitus, diabetic retinopathy and diabetic neuropathy. J Res Med Sci 2014;19(11):1090-8.
Kamiyama M, Urushihara M, Morikawa T, Konishi Y, Imanishi M, Nishiyama A, et al. Oxidative stress/angiotensinogen/renin-angiotensin system axis in patients with diabetic nephropathy. Int J Mol Sci 2013;14(11):23045-62. https://doi.org/10.3390/ijms141123045.
Urushihara M, Kagami S. Urinary angiotensinogen as a biomarker of nephropathy in childhood. Int J Nephrol 2011;2011:206835. https://doi.org/10.4061/2011/206835.
Ewens KG, George RA, Sharma K, Ziyadeh FN, Spielman RS. Assessment of 115 candidate genes for diabetic nephropathy by transmission/disequilibrium test. Diabetes 2005;54(11):3305-18. https://doi.org/10.2337/diabetes.54.11.3305.
Rizvi S, Raza ST, Mahdi F. Association of genetic variants with diabetic nephropathy. World J Diabetes 2014;5(6):809-16. https://doi.org/10.4239/wjd.v5.i6.809.
Gao T, Huang L, Fu Q, Bai Y. Association of polymorphisms in the AGT gene (M235T, T174M) with ischemic stroke in the Chinese population. J Renin Angiotensin Aldosterone Syst 2015;16(3):681-6. https://doi.org/10.1177/1470320315583600.
Gaillard I, Clauser E, Corvol P. Structure of human angiotensinogen gene. DNA 1989;8(2):87-99. https://doi.org/10.1089/dna.1.1989.8.87.
Moon JY. Recent update of renin-angiotensin-aldosterone system in the pathogenesis of hypertension. Electrolyte Blood Press 2013;11(2):41-5. https://doi.org/10.5049/EBP.2013.11.2.41.
Brand E, Chatelain N, Paillard F, Tiret L, Visvikis S, Lathrop M, et al. Detection of putative functional angiotensinogen (AGT) gene variants controlling plasma AGT levels by combined segregation-linkage analysis. Eur J Hum Genet 2002;10(11):715-23. https://doi.org/10.1038/sj.ejhg.5200874.
Sethi AA, Nordestgaard BG, Tybjaerg-Hansen A. Angiotensinogen gene polymorphism, plasma angiotensinogen, and risk of hypertension and ischemic heart disease: A meta-analysis. Arterioscler Thromb Vasc Biol 2003;23(7):1269-75. https://doi.org/10.1161/01.ATV.0000079007.40884.5C.
World Health Organization. Definition, diagnosis and classification of diabetes mellitus and its complications. Report of a WHO Consult. Part 1: Diagnosis and Classification of Diabetes Mellitus. Geneva: WHO Department of Noncommunicable Disease Surveillance; 1999. p. 1-59.
Elston RC, Forthofer R. Testing for Hardy-Weinberg equilibrium in small samples. Biometrics 1977;33(3):536-42. https://doi.org/10.2307/2529370.
Norton GR, Brooksbank R, Woodiwiss AJ. Gene variants of the renin-angiotensin system and hypertension: From a trough of disillusionment to a welcome phase of enlightenment? Clin Sci (Lond) 2010;118(8):487-506. https://doi.org/10.1042/CS20090498.
Underwood PC, Sun B, Williams JS, Pojoga LH, Raby B, Lasky-Su J, et al. The association of the angiotensinogen gene with insulin sensitivity in humans: A tagging single nucleotide polymorphism and haplotype approach. Metabolism 2011;60(8):1150-7. https://doi.org/10.1016/j.metabol.2010.12.009.
Prasad P, Tiwari AK, Kumar KM, Ammini AC, Gupta A, Gupta R, et al. Chronic renal insufficiency among Asian Indians with type 2 diabetes: I. Role of RAAS gene polymorphisms. BMC Med Genet 2006;7:42. https://doi.org/10.1186/1471-2350-7-42.
Ahluwalia TS, Ahuja M, Rai TS, Kohli HS, Bhansali A, Sud K, et al. ACE variants interact with the RAS pathway to confer risk and protection against Type 2 diabetic nephropathy. DNA Cell Biol 2009;28(3):141-50. https://doi.org/10.1089/dna.2008.0810.
Shaikh R, Shahid SM, Mansoor Q, Ismail M, Azhar A. Genetic variants of ACE (insertion/deletion) and AGT (M268T) genes in patients with diabetes and nephropathy. J Renin Angiotensin Aldosterone Syst 2014;15(2):124-30. https://doi.org/10.1177/1470320313512390.
Mtiraoui N, Ezzidi I, Turki A, Chaieb M, Mahjoub T, Almawi WY. Renin-angiotensin-aldosterone system genotypes and haplotypes affect the susceptibility to nephropathy in type 2 diabetes patients. J Renin Angiotensin Aldosterone Syst 2011;12(4):572-80. https://doi.org/10.1177/1470320310396542.
Chang HR, Cheng CH, Shu KH, Chen CH, Lian JD, Wu MY. Study of the polymorphism of angiotensinogen, anigiotensin-converting enzyme and angiotensin receptor in type II diabetes with end-stage renal disease in Taiwan. J Chin Med Assoc 2003;66(1):51-6.
Osawa N, Koya D, Araki S, Uzu T, Tsunoda T, Kashiwagi A, et al. Combinational effect of genes for the renin-angiotensin system in conferring susceptibility to diabetic nephropathy. J Hum Genet 2007;52(2):143-51.
https://doi.org/10.1007/s10038-006-0090-5.
Wang J, Zhu X, Yang L, Liu Y, Zhou W, Li H. Relationship between angiotensinogen gene M235T variant with diabetic nephropathy in Chinese NIDDM. Chin Med J (Engl) 1999;112(9):797-800.
Reis KA, Ebinç FA, Koç E, Demirci H, Erten Y, Güz G, et al. Association of the angiotensinogen M235T and APO E gene polymorphisms in Turkish type 2 diabetic patients with and without nephropathy. Ren Fail 2011;33(5):469-74. https://doi.org/10.3109/0886022X.2011.568133.
Su SL, Yang HY, Wu CC, Lee HS, Lin YF, Hsu CA, et al. Gene-gene interactions in renin-angiotensin-aldosterone system contributes to end-stage renal disease susceptibility in a Han Chinese population. ScientificWorldJournal 2014;2014:169798. DOI: 10.1155/2014/169798.
Ding W, Wang F, Fang Q, Zhang M, Chen J, Gu Y. Association between two genetic polymorphisms of the renin-angiotensin-aldosterone system and diabetic nephropathy: A meta-analysis. Mol Biol Rep 2012;39(2):1293-303.
https://doi.org/10.1007/s11033-011-0862-7.
Buraczynska M, Ksiazek P, Lopatynski J, Spasiewicz D, Nowicka T, Ksiazek A. Association of the renin-angiotensin system gene polymorphism with nephropathy in type II diabetes. [Article in Polish]. Pol Arch Med Wewn 2002;108(2):725-30.
Ringel J, Beige J, Kunz R, Distler A, Sharma AM. Genetic variants of the renin-angiotensin system, diabetic nephropathy and hypertension. Diabetologia 1997;40(2):193-9. https://doi.org/10.1007/s001250050662.
Campbell CY, Fang BF, Guo X, Peralta CA, Psaty BM, Rich SS, et al. Associations between genetic variants in the ACE, AGT, AGTR1 and AGTR2 genes and renal function in the Multi-ethnic Study of Atherosclerosis. Am J Nephrol 2010;32(2):156-62. https://doi.org/10.1159/000315866.
- XML
How to Cite
1.
Makuc J, Šeruga M, Završnik M, Cilenšek I, Petrovič D. Angiotensinogen (AGT) gene missense polymorphisms (rs699 and rs4762) and diabetic nephropathy in Caucasians with type 2 diabetes mellitus. Bosn J of Basic Med Sci [Internet]. 2017Aug.20 [cited 2021Dec.21];17(3):262-7. Available from: https://www.bjbms.org/ojs/index.php/bjbms/article/view/1823
Section
Translational and Clinical Research
INTRODUCTION
Diabetic nephropathy (DN) is a chronic, progressive microvascular complication of diabetes mellitus. It is associated with high cardiovascular morbidity and mortality and is still the most common cause of end-stage renal disease (ESRD) in developed countries [1-4]. DN is the result of interaction between environmental and genetic factors, where the letter are relatively common and probably linked genes, and these factors also include related epigenetic mechanisms [1,5,6]. Clinically, non-pharmacological interventions such as strict glycemic and blood pressure control, non-smoking habits, and dietary protein intake have been shown to slow the progression of DN. Nevertheless, the most common clinical strategy for slowing the disease progression is therapeutic targeting of the renin–angiotensin–aldosterone system (RAAS) [2-4,7,8]. This is related to the pathophysiological relationship between DN and increased blood pressure, i.e. type 2 diabetes mellitus (T2DM) and DN are usually associated with hypertension [2,3,7]. The complex underlying mechanisms are still not completely understood and include excess sodium retention, sympathetic nervous system activation, endothelial cell dysfunction, increased oxidative stress, and RAAS activation [7]. All components of the systemic RAAS are also present in local (tissue) RAAS, and for example in the kidneys, the local effects accelerate the progression of renal disease [7,9]. Data suggest that the activation of intrarenal angiotensinogen (AGT) production plays an important role in the development of DN [10]. Urinary AGT was proposed as a possible early marker of DN, as it reflects intrarenal RAAS status in chronic glomerulonephritis [11].
Although RAAS gene polymorphisms have been extensively studied in diabetic and cardiovascular diseases, including several meta-analyses, contradictory results were reported [9,12-15]. AGT gene is located on the long arm of chromosome 1 (1q42-43), consisting of five exons and four introns [16]. AGT codes for 485 amino acids (AA), including: A signal peptide (AA 1-33), AGT chain (AA 34-485) and several other peptides, such as angiotensin I-III, which are all part of the RAAS. In brief, AGT is a plasma globulin belonging to the serpin family, synthetized in the liver. It is cleaved by renin which produces inactive decapeptide angiotensin I. Angiotensin-converting enzyme (ACE) converts angiotensin I to active octapeptide angiotensin II which increases aldosterone secretion, elevates blood pressure, and inhibits renin secretion by binding to angiotensin II receptors. ACE II forms angiotensin (1-7) from angiotensin II which has vasodilatory, antiproliferative, and apoptotic functions [17].
In this study, we investigated the possible effects of two missense single nucleotide polymorphisms (SNPs) of the AGT gene on DN. SNP rs699 is a T to C substitution in the exon 2, resulting in a functional methionine (M) to threonine (T) exchange at codon 268 (M268T). Previously, rs699 was positioned to the amino acid 235 and the SNP is therefore also referred to as M235T. The rs699 threonine variant is associated with higher plasma AGT levels and higher blood pressure [18,19].
SNP rs4762 is a C to T substitution in AGT exon 2 with a consequent functional threonine (T) to methionine (M) exchange at codon 207 (termed T207M or T174M). This polymorphism was not associated with higher plasma AGT levels [18].
The aim of our study was to examine the association between the AGT rs699 and rs4762 polymorphisms and DN in Caucasians with T2DM.
MATERIALS AND METHODS
Patients
A total of 651 consecutive, unrelated Slovenian (Caucasian) T2DM patients from outpatient clinics of the University Medical Centre Maribor, General Hospitals Murska Sobota and Slovenj Gradec, Slovenia, were enrolled in the study in the period from 2010 to 2014. The T2DM patients were classified into two groups according to the presence of DN. A study group consisted of 276 T2DM patients with DN (DN+, cases), while control group included 375 patients without DN but who have had T2DM for >10 years (DN−, controls). Due to the cross-sectional study design, the cases and controls were not matched for their demographic, lifestyle, or medical characteristics.
The diagnosis of T2DM and DN was made according to the World Health Organization diagnostic criteria [20]. Patients with overt nephropathy, poor glycemic control, significant heart failure (New York Heart Association [NYHA] II-IV), alcoholism, infection, and other causes of renal disease were excluded.
The study was approved by the national medical Ethics Committee and was performed in compliance with the Helsinki declaration. All patients were interviewed in person for lifestyle and medical information after signing an informed consent to participate in the study. The questions concerned age, sex, blood pressure, duration of T2DM and hypertension, body mass index (BMI), smoking status, incidence of microvascular complications of T2DM (diabetic retinopathy [DR], DN, diabetic foot [DF]), duration of DR, estimated glomerular filtration rate (eGFR), hemoglobin (Hb), total cholesterol, high-density lipoprotein (HDL) and low-density lipoprotein (LDL) cholesterol levels.
Biochemical analyses
Plasma glucose, Hb, glycated hemoglobin (HbA1c), urea, creatinine, cystatin C, total cholesterol, LDLs, HDLs, and triglycerides (TGs) were determined by standard biochemical methods. For each patient, albumin/creatinine ratio (ACR) was also determined in three urine samples according to diagnostic criteria.
Genotyping
Genomic DNA was extracted from 100 μl of peripheral blood using a Qiagen isolation kit (DNeasy Blood and Tissue Kit, Qiagen, Germany). The rs699 and rs4762 polymorphisms of the AGT gene were genotyped by LGC – Genomics Laboratories (Registered Office: LGC, Queens Road, Teddington, Middlesex, TW11 OLY, UK) using their novel fluorescence-based kompetitive allele-specific polymerase chain reaction [PCR] (KASPar) assay. The details of this method can be found at http://www.kbioscience.co.uk/. The following primers (5'-3') were used for PCR reaction:
-
AGT (rs699)/F: CAGGGTGCT GTCCACACTGG ACCCC
-
AGT (rs4762)/R:CCGTTT GTGCAGGGCCTGG CTCTCT.
Statistical analysis
Statistical analyses were conducted using SPSS program for Windows version 20 (SPSS Inc., Illinois, USA). The distribution of data was analyzed by the Kolmogorov–Smirnov test. Continuous variables were expressed as mean ± standard deviation (SD) when normally distributed, and compared by unpaired Student's t-test. The variables were expressed as median values (interquartile range [IQR]) when asymmetrically distributed, and compared using the Mann–Whitney U test. Chi-square test was used to compare discrete variables. Categorical variables were expressed as the number and percentage of patients. Furthermore, all variables that showed significant differences by the univariate analysis were analyzed together in a logistic regression analysis (for both analyses p < 0.05 was considered statistically significant). The deviation from Hardy-Weinberg equilibrium (HWE) was assessed by the exact test (http://ihg.gsf.de/) [21].
RESULTS
The population characteristics are presented in Table 1. There were no significant differences between DN+ and DN− groups with respect to the age, sex, duration of T2DM, diastolic blood pressure (DBP), BMI, smoking status, duration of DR, eGFR, Hb, total cholesterol, HDL, and LDL cholesterol levels. However, there was a statistically significant difference in the duration of hypertension, systolic blood pressure (SBP), family history of cardiovascular disease (CVD), urine ACR, HbA1c, TG, fasting glucose, urea, and creatinine levels. Moreover, in DN+ group, a higher percentage of other chronic diabetic complications (i.e., DR and DF) was found compared to the patients without DN (Table 1).
TABLE 1: Population characteristics of T2DM patients with DN (DN+, cases) and patients without DN but who have had T2DM for >10 years (DN−, controls)
Differences in parameters of kidney function (i.e., serum urea, creatinine, cystatin C, and urine ACR) were consistent with chronic kidney disease in DN+ group. These patients showed significantly more other chronic diabetic complications, such as DR and DF, and were more burdened with CVD, although no differences were found regarding their family history of CVD, smoking status, lipid profile, or diabetes duration. Importantly, they had worse glycemic control, higher SBP, and longer history of hypertension compared to the patients without DN (Table 1).
The genotype distribution and allele frequencies of AGT rs699 and rs4762 polymorphisms in DN+ and DN− groups are presented in Table 2. No significant differences in the allele frequencies were found between the two groups. In both groups, rs699 and rs4762 SNPs conformed to the HWE (DN+ group: rs699, p = 0.43; rs4762, p = 0.28; DN− group: rs699, p = 0.47; rs4762, p = 0.58).
TABLE 2: Distribution of AGT rs699 and rs4762 genotypes and alleles in patients with diabetic nephropathy (cases) and in those without diabetic nephropathy (controls)
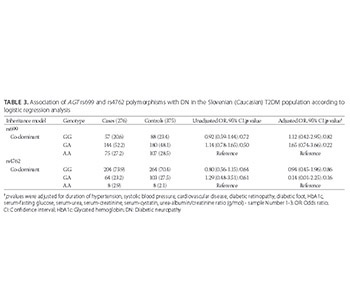
Furthermore, no association between the two SNPs and DN was found in the multivariate logistic regression analysis adjusted for different confounders (i.e., duration of hypertension, SBP, CVD, DR, DF, HbA1c, fasting glucose, urea, creatinine, cystatin C, urine ACR), according to the co-dominant genetic model (Table 3).
TABLE 3: Association of AGT rs699 and rs4762 polymorphisms with DN in the Slovenian (Caucasian) T2DM population according to logistic regression analysis
DISCUSSION
In our study DN+ patients showed higher CVD morbidity compared to the controls, despite adjusted CVD family burden; furthermore, they showed higher BMI, and differences with regard to the smoking habits and lipid profile were also observed between the two groups. Hypertension contributes to the increased incidence of CVD in patients with DN [7]; therefore, blood pressure control is of special interest in these patients. There is also a relationship between T2DM, DN, and increased blood pressure. The latter is commonly present in patients with T2DM before DN development and leads to further progression of kidney disease in existing DN. Taking into account the beneficial effects of RAAS therapeutic targeting [2-4,7,8], the effect of RAAS gene polymorphisms on the development and progression of DN has been in focus. The RAAS genes have been studied extensively in both hypertension and control patients, with several well-designed meta-analyses that both confirmed and opposed the role of the RAAS gene polymorphisms in this condition [21].
Numerous studies also investigated the role of AGT rs699 polymorphism (M235T, M268T) in DM and chronic diabetic complications. Although an association with insulin resistance [22], serum AGT and blood pressure was noted [18,19], a systematic review of 73 studies by Rahimi et al. [9] indicated that this polymorphism does not affect the risk of DM and could not be associated with DR nor diabetic neuropathy [9]. In certain populations, the association between increased risk of DN and the polymorphic allele T or genotype TT was demonstrated (i.e., in two Indian populations [23,24], a Pakistani [25], Tunisian [26], Taiwanese [27], Japanese [28], Chinese [29], and Turkish population [30]). However, the reviews and extensive meta-analyses could not confirm the association of rs699 with DN [13,14,31,32]. Our results match these findings as we found no association between rs699 and DN in the T2DM population, and they also resemble the results obtained for Polish [33] and German population [34].
In contrast to rs699, AGT rs4762 polymorphic variant (T207M, T174M) was not included in the meta-analyses, as most individual studies did not show the association of rs4762 with DN [25,27] nor with ESRD [31]. The association of rs4762 with DN was only confirmed in Taiwanese population [27]. In addition, rs4762 was not associated with plasma AGT levels [18]. Similarly, we found no association between rs4762 and DN in our population.
Previous studies suggested that the association of AGT polymorphisms with different phenotypes varies across racial/ethnic groups [22,35], and this could potentially explain the negative results obtained in our study. Nevertheless, a meta-regression analysis was performed to evaluate the effect of ethnic heterogeneity on the results of association studies for various genes. They concluded that the lack of association of several genes (but not the AGT) with DN could be explained by ethnic heterogeneity [13]. Our results could also be related to the fact that DN is not the only cause of kidney disease in DM patients. Moreover, although renal biopsy is the gold standard for the diagnosis of DN, it is not commonly performed in DM patients. Instead, the diagnosis of DN is based on a combination of kidney disease and other specific characteristics [7,20]. Finally, our results are most likely the consequence of DN being a polygenic trait. Similarly, the results of other studies that identified genes or genome regions associated with DN were quite inconsistent [5]. Thus, the focus should not only be on single polymorphisms when investigating genetic basis of DN. In addition, epigenetic mechanisms are also involved in DN development (e.g., DNA methylation, chromatin histone modifications, and functional noncoding RNAs [6]), and therefore should be considered in future studies.
CONCLUSION
AGT rs699 and rs4762 missense polymorphisms are not associated with DN in our subset of Slovenian T2DM patients. Overall, our results contribute, at least partially, to better understanding of the genetic background of DN.
DECLARATION OF INTERESTS
The authors declare no conflict of interests
American Journal of Medical Genetics Doi 10.1002 Ajmg.a
Source: https://www.bjbms.org/ojs/index.php/bjbms/article/view/1823
Posted by: perrodinshandid.blogspot.com

0 Response to "American Journal of Medical Genetics Doi 10.1002 Ajmg.a - perrodinshandid"
Post a Comment